Eye Drops Ready for Licensing-out
Developed and produced in EU.
25 years of expertise and experience in ophthalmology.
Developed and produced in EU.
25 years of expertise and experience in ophthalmology.


A range of eye drops to help relieve dry eye symptoms.
Compatible and safe to use with contact lenses.
Complementary ingredients work in perfect synergy to deliver superior performance.
moisturising and quick relief for dry eye symptoms
regeneration of micro damages of eye surface

Indication
For the relief of moderate to severe symptoms of dry eye disease (DED). Restores the lipid layer of the tear film.
Composition
Sodium hyaluronate 0,15%, glycerol 1%, castor oil 0,25%, vitamin E
Number of drops
320
Pharmaceutical form
Eye drops 10 ml, emulsion
Dossier available
According to EU medical device regulation
Regulatory classification
Medical device

Indication
Relief of mild dry eye problems
Composition
Sodium hyaluronate 0,21%
Number of drops
350
Pharmaceutical form
Eye drops 10 ml, solution
Dossier available
According to EU medical device regulation
Regulatory classification
Medical device

Indication
Relief of severe dry eye problems
Composition
Sodium hyaluronate 0,4%
Number of drops
350
Pharmaceutical form
Eye drops 10 ml, solution
Dossier available
According to EU medical device regulation
Regulatory classification
Medical device
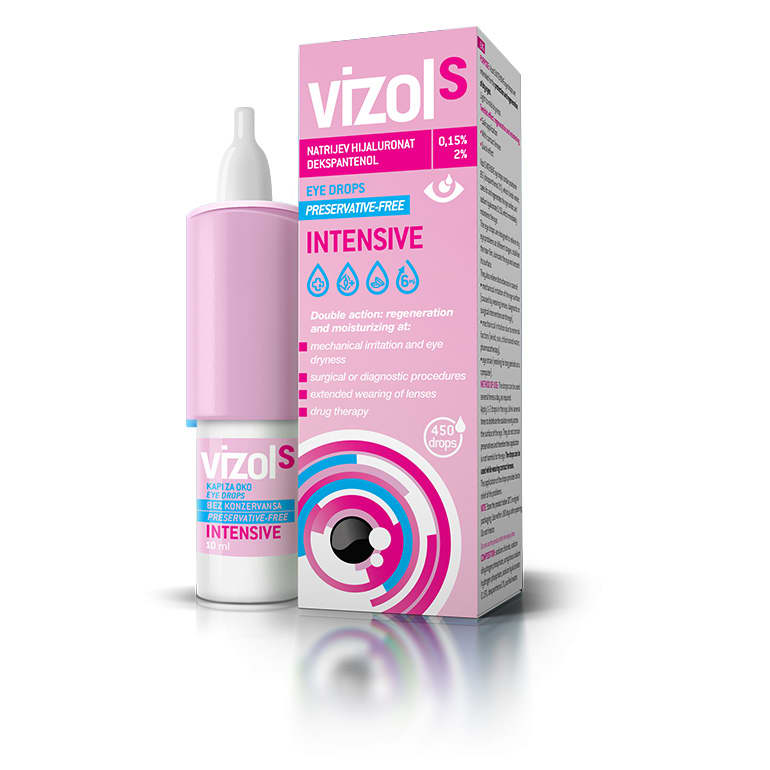
Indication
Relief of dry eye problems and regeneration of eye surface
Composition
Sodium hyaluronate 0,15% + dexpanthenol 2%
Number of drops
450
Pharmaceutical form
Eye drops 10 ml, solution
Dossier available
According to EU medical device regulation
Regulatory classification
Medical device
Pharmaceutical form
Eye drops, solution
Period after opening
6 months
Shelf life
36 months
Eye drops to help relieve eye redness and allergic eye inflammation.
Eye redness, unrelated to infection or injury, is a common condition that can be caused by a variety of conditions as eye fatigue, dryness or dust, overexposure to the sun, allergies, swimming in chlorinated pools, or contact lenses.
Contact lenses friendly.
The only eye drops for red eye without preservatives!
After applying locally, the vasoconstrictive and decongestive effect of tetryzoline begins after a few minutes and lasts for 4 to 8 hours.

Indication
Relief of eye redness and allergic eye inflammation
Composition
Tetryzoline hydrochloride, 0.5 mg/ml
Pharmaceutical form
Eye drops 10 ml, solution
Dossier available
eCTD, the 1st PF formulation registered in EU
Regulatory classification
OTC drug
Pharmaceutical form
Eye drops, solution
Period after opening
28 days
Shelf life
30 months
We nurture collaboration with renowned pharmaceutical companies based on a strategic approach, flexibility, top professionalism and extensive experience.
Necessary cookies enable core functionality. The website cannot function properly without these cookies, and can only be disabled by changing your browser preferences.